Throughout the year I have learned and explored many new consepts on the world of science and chemistry. I have learned many chemistry theorises and big ideas. Many labs and lectures have got me thinking very differently about scince. It all began with the Scientific Observations Lab. Learning the techniques and recording the observatiosn in a notebook. Making specific descriptions on what is happening in the lab. The lab techniques lab werwe helpful in kniowing hoew to handle chemicals. It was also a good way to learn about the variety of chemicalsand how to work with them and percautions one must take when handling different chemicals. Another impostant lab to help me learn the ways of carrying out a lab was the Periodic Table Trends Lab. I got to explore the elements on the Periodic Table and the patterns on the Periodic Table. For exple the patterns of atomic radius, ionization energy, and electronegativity. The Determining Percent of a Compound in a solid was also a important lab because we were able to determine the persent of a compound in a solid. The Candium Lab may have seen silly using m&m’s but it acually helped me more than one would think. This lab helped me to understand the radioactive decay and understanding the different amounts of radioactive and decayed elements. The chemical Bonding Lab was also a very important lab. Comparing the melting points of solids and determining the solubilities and classifing the into groups of ionic and covalent compounds really helped me understand more on the world of science. The law of Conversation of Mass Lab was helpful to learnand prove the law of converstaion of mass. The ice melt lab was helpful to learn how to crate a graoh on the results that I got during the lab. Boiling ater and writing down the results was boring but it was helpful in the end to improve my lab notes. The Ideal Gas Law Lab was interesting because we got to use new matierals in the lab station. The colligative properties Lab was fun because we got to eat ice cream. It was cool to make it without a frezzer and all. The ice cream tasted very good and it was cool that I was able to make it myself. It was very intersting that the ice and the salt in the bag could freeze the ice cream enough to become less liqidy. The acid- base titration lab was good to learn the basic labratory technique of titration and calculating the molality based on titrations. Learning about the acid and base rules and the pH stuff was difficult, so doing this lab gave me a better understanding and allowed me to pratice how to find molality. Stochiometry was a difficult thing to learn. I would get very confused on where to put what number and when to multiply, divide and add and subtract all the different numbers. Now at the end of the year I can say that I was able to learn the process of Stochiometry. This year has been a great learning experience and I am glad I will have the information I have learned for the rest of my life.
Monday Blog
Recently I read an article on the melting glaciers in Antartica. Scientist have learned that it may be that the undersides of the ice sheets are responsible for the worlds ice loss. I learned that ice shelves is a floating sheet of ice permanently attached to a landmass, http://www.esr.org/outreach/glossary/ice_shelf.html. I also learned the term propulsion which is the the process of driving or propelling, http://www.thefreedictionary.com/propulsion. The global warming of the ocean is making the ice sheets under the ocean melt. There are more issues that follow with this melting of ice sheets in Antarctica. This is the original article, http://www.sciencedaily.com/releases/2013/06/130613142827.htm. For more interesting articles on this topic http://www.sciencedaily.com/news/earth_climate/global_warming/.
Soap LAb
Throughout this blog I will tell you how to make soap with these instructions.
Anytime in the lab area saftey goggles, aprons, and gloves should be used. Also tie hair back and wear apropriate shoes. 
Next thing to do is to get all your ingrediates together for the lab.
now its time to begin the soap making progress!!
Step one:measure out 250ml of water into erlenmeyer flask
Step 2: mass NaOH in a weigh boat (make sure not to touch)
Step 4: mass 1g of sugar and add it to the erlenmeyer flask
Step 5: melt oils if necessary, then measure, and combine all oils into a 400mL beaker.
Step 6:next slowly poor the two mixtures together, stirring throughout the process.
Step 7: Keep stirring to create a thick paste. This could take a while so it is suggested to use a handheld emulsifier.
this also can be a good time to add a color or scent.
Step 8: pour the trace into a mold then wait 4-6 weeks to harden and mature to lose its alkalinity.
Step 9: Clean up using water and return matierals and wipe down lab station.
Step 10: USe your soap and experiment with different colors and scents and molds next time!!
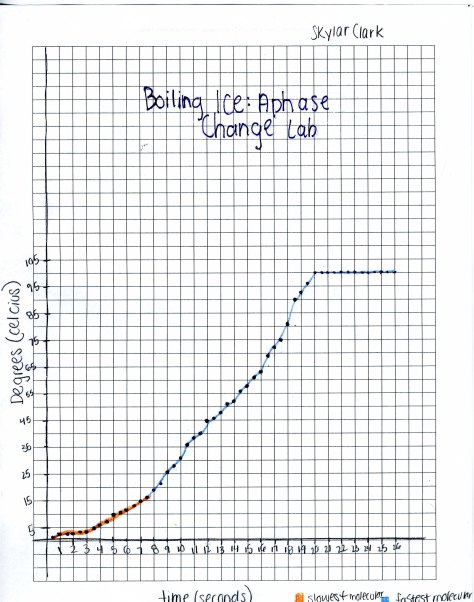
Boiling Ice: A Phase Change Lab
Boiling Ice: A Phase Change Lab
Throughout this lab ice was melting. As the temperature raised the ice melted faster. At the top of my graph where the line is straight the temperature stayed at the constant temp of 100. At that point all the ice had melted and it was gone. Molecular motion is taking place. The ice is absorbing heat. When the temp got to 17 Celsius thew molecular motion got faster. It was very slow moving before that point. This lab helped to see the how molecules turn with the presence of heat.
I Used to think… But Now I think…
I used to think that gases was just the stuff we breath and the things inside a balloon. Also that it had to do with things when the boiled or when climbing a mountain. Now I think that I know what the gases actually do. I know that they can expand and deflate when it is hotter or colder. There is a large world of gases that we all live in.
Law of Conservation of Mass
January 14, 2013
Class D
Date lab was performed: January 8,2013
LAW OF CONSERVATION OF MASS
- Law of Conservation of Mass is a relation stating that in a chemical reaction, the mass of the products equals the mass of the reactants.
- The real mass changes and apparent ones due to the limitations of the balance are distinguished by the chemicals added to the substance. In this lab lead nitrate is added with potassium iodide. The apparent masses are the ones that are obvious. One may think that it has changed but it really has not.
- When I mixed the solutions in activity one, the change my partner and I observed did not affect the mass.
- In activity two, when the vinegar and baking soda combined it got lighter and made the Ziploc bag blow up. This did result in a change of mass in the open bag. This change resulted in change of mass in the closed flask because the baking soda was loose in the bag. Accounting for the changes in mass can be done by making sure to weigh everything very carefully.
- The heating of the steel wool in activity 3 affected its appearance by making it appear blue. This did not result in a change of mass. I could account for this because I weighed it before and after.
- Massing every material and doing accurate measurements the apparent changes in mass can be proved or disproved.
- Making sure all materials are massed correctly before doing anything with them it can modify such experiments.
What I learned: Throughout these activities I learned about the law of conservation of mass. Also I learned to be careful when massing materials before and after and throughout a experiment. Also I learned to account for the changes in mass between the differences of when mixing or burning substances. Learning these things will help me be a better scientist.
Activity one: lead nitrate
+ potassium iodide
35.53g(before and after mixing)
Activity two: Ziploc and vial w/lid= 113.12
Ziploc opened vial = 111.59
Activity three: steel wool(before and after burning)= 1.72g
Safety in the Science Classroom
Safety of yourself and others while conducting an experiment is very important. You have responsiblility of not only yourself but everyone around you. Following the directions of an experiment is very important. If one thing goes wrong, you could end up in a very dangerous situation. Having the directions where you can access them could be helpful. Putting correct footwear and glasses, and aprons and gloves if needed is essential as well. Also another crucial safety feature would be to have access to an eye wash station, emergency shower, a fire blanket, fire extinguisher, and a first aid kit. Eye wash stations are used when one gets a irratating chemical in their eye and they need to flush whatever it is out imediatly. Emergency showers are for getting something off the skin. Fire blankets would be used if one was on fire and the blanket would put out the fire. Fire extinguishers would be used in case of a fire in a classroom, but only if necessary. It is much safer to exit the classroom or lab staion and get somewhere safe. First aid kits would be used for smaller cuts or bruises. Saftey in the lab area is necessary to make everything go smoothly, safe, and fun!
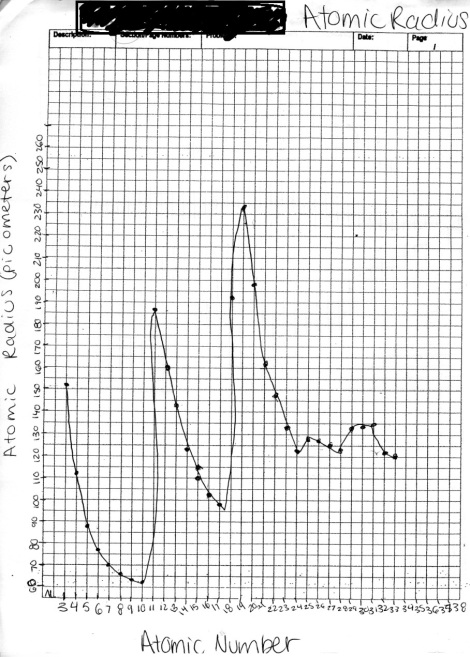
How do periodic trends relate to the electron structure of an element?
Each electron in an atom is described by different quantum numbers. In a single electron atom or hydrogen atom, n would be the only quantum number which determines each electron in an atom is described by different quantum numbers. In a single electron atom or hydrogen atom, n would be the only quantum number which determines the energy level. It also determines the size of a orbital. energy level. It also determines the size of a orbital. As you go down the periodic table by the colomns, the atomic radius increases, because of the excess protons gaining in the valence bond.
How do we know what we know about the building blocks of matter?
Previous Scientist have given the scientist of today a great outlook on the building blocks of matter. Atoms have been the most frequently used building blocks of matter. Many other wonderful building blocks of matter have been found to create a scientific world. The elctron configuration effects the behavior of an element by giving off wave lenth. The octet rule is when it wants eight electrons in its outmost energy level. This is when it is at its happiest.Avogadro’s number also helps scientist to have a common number they can use when doing math between atoms. The periodic table of elments also is used by many scientist to help them have a consistent number to use.
Life as a Scientist
Hi World! I am a student in a chemistry class and I perform experiments every week. It is so cool learning new things about the possibilties of the world. I enjoy making connections to other things in life through the labs we do in class. The labs we do always seem to amaze me and it interests me how everything in this world connects to something. I cant wait to see what else I can learn in the science world!